
This is because the molecules are larger, and therefore have more mass. The density of a gas also increases as you go down the group. The larger the molecule, the higher the boiling point. This is because the molecules are larger, and therefore have more electrons. The boiling points of the noble gases increase as you go down the group.
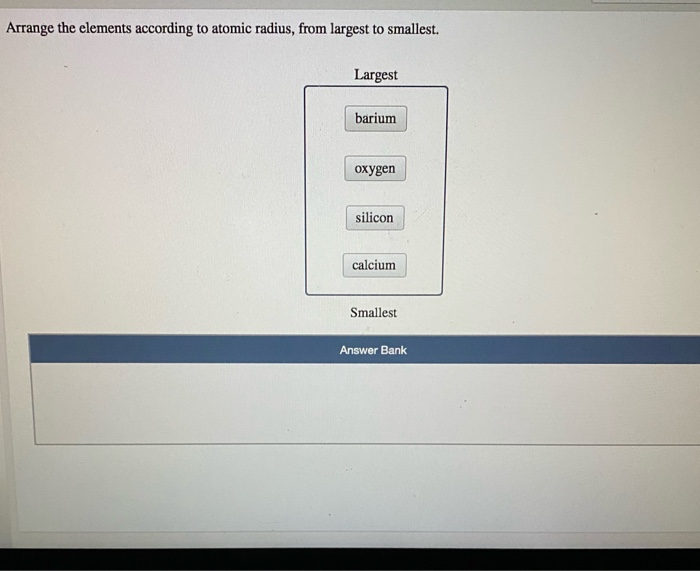
Largest to smallest atomic radius full#
All of the noble gases are noble because they have full valence electron shells. The lightest noble gas is helium, and the heaviest is radon. They are all colorless, odorless, and tasteless. Group 0 elements are called the noble gases because they are all monatomic. By the time we get to radon, the outermost orbital is so diffuse that the atom has actually increased in size compared to xenon. Because additional electron shells are progressively built, atomic radii always improve as a result of adding extra electrons to an atom’s valence shell. The atomic radii of group 0 noble gases rise down the column. The density of group 0 noble gases goes up with each increase in group number. The boiling point of a group 0 noble gas rises as the group number increases.
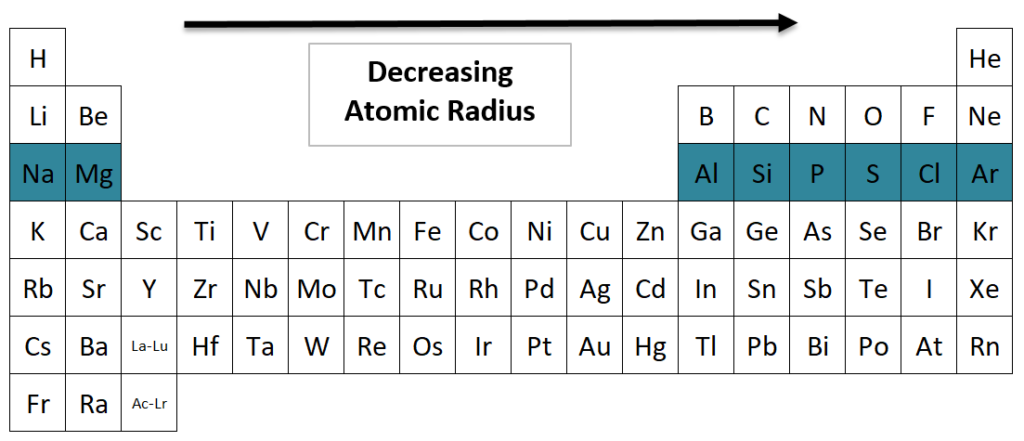
The atomic radius of a neon atom is smaller than that of the fluoride ion (F−), for example. The repulsive forces between the electrons in noble gas atoms cause them to move closer to the nucleus, despite their proximity. Why do noble gases have smallest atomic radius? As a general rule, elements on the left side of the periodic table have smaller atoms than those on the right side. One way to determine the atomic radius of an element is by looking at the atomic radius trend in the periodic table. This is why noble gases have no electronegativity. Noble gases already have a full valence shell of electrons and are stable, so they do not need to attract them in order to be electronegative. The atom’s capacity to attract electrons within a connection is referred to as electronegativity. Why do noble gasses have no electronegativity? Due to an increase in the nucleus’ attractive force, atomic size decreases from left to right as you go left to right across the periodic table. (iv) Because both potassium and calcium are in the third period of the periodic table, their radii are smaller. Why is the atomic radius of calcium smaller than potassium? As a result, helium is the tiniest element, while francium is the most massive. The atomic radius rises from top to bottom in a group and decreases from left to right across a period, as shown in the figures below. The atomic radii of the various elements vary in a repeating pattern across the periodic table. What element has the largest atomic radius? Because of the nuclear charge, the atomic radius decreases from left to right. Helium is the lightest noble gas, with an atomic radius of 0.000393584 nm. Which is the noble gas with smallest atomic radius? The subtlety here is that this trend does not always hold perfectly true, especially for the noble gases. This is because the electron clouds become bigger and more diffuse, leading to a greater atomic radius. Do atoms get bigger as you go down a group?Ītoms generally get larger as you go down a group. This is due to trends in the periodic table, as well as a strong nuclear charge that keeps the valence electrons close to the nucleus. The atomic radius of helium is the smallest.

As a consequence, the period when noble gases reign supreme is lasting. As a result of this, the vanderwaals radius is always greater than the covalent radius. Why do noble gases have larger atomic radii? Since they have completely full octets, electron-electron repulsion is greater in them. Do noble gases have the largest atomic radius? The reason for this is that these atoms have very little interatomic interactions because they have complete valence shells. The radius of a noble gas atom is generally small. The idea behind this is that noble gases have little chemical activity and thus their atomic radii are not joined. As a result, only Van der Waals radius is measured. Noble gases are considered to be non-bonding and non-forming. The reason that noble gases have the smallest atomic radius is because they are at the end of the periodic table. Why do noble gases have the smallest atomic radius? However, there are some exceptions such as Francium, which is the largest element due to its high number of electrons. This is due to the fact that nuclear radii decrease down a period and increase in a group, which is why Noble gases are the final group and HELIUM, being the top element, has the greatest atomic radius among noble gases. Noble gases have the smallest atomic radius of all HELIUM.


 0 kommentar(er)
0 kommentar(er)
